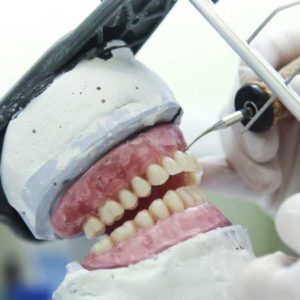
FEPPD, the European Federation of Dental Labs and Dental Technicians is contacting you to share important information regarding the uniform interpretation of the status of “manufacturer” in the scope of the Medical Devices Regulation. The FEPPD Board, has been following for quite some time, rumours coming from the dentist’s lobby with regards to their unwillingness...
NewsEuropean Dental Technicians Day 2019.
Catégorie : MDR
Article
Dental technicians & Medical Device Regulation
The European Commission, at the request of the European Federation of Dental Technicians and Lab owners, specified that a custom made dental device for the single use of a patient, remains subject to the requirements of the regulations on medical devices in terms of quality and traceability of materials, regardless of whether it is manufactured...
Article
Medical Device Regulation adopted by European Parliament
The European Parliament has adopted the Medical Device Regulation (‘MDR’) during its second reading on April 5th, 2017 (see press release attached). The Parliament’s vote constitutes the final step in the legislative process for the adoption of the MDR and confirms the informal political agreement that was reached with the Council and the Commission in...